Surgical FaceMasks
The mdd surgical FaceMask is developed to prevent any transmission of airborne infectious agent. The hig performance tested under the EN 14683:2019 confirm the efficacy of our facemask in professional areas
Classification
Medical device
EN 14683:2019 Type IIR
Standards
EN 14683:2019.
Additional standards:
EN 149:2001+A1:2009
EN 1041:2008
EN ISO 10993-1:2009
EN ISO 10993-5:2009
EN ISO 10993-10:2010
ISO 15223-1:2016
EN ISO 14971:2012
Size
17×9.5 – Flat shape
Use
The mdd surgical Face Masks
Are projected to avoid droplet spreading secreted by human saliva and to prevent the transmission of pathogens.
The user of the Face Mask can be a healthcare professional or a patient who wants to protect themselves or others from transmission of pathogens.
Wear time
Dispose of after single use
Storage
In the original packaging, cool and dry, avoid the direct sunlight
Disposal
Safe thermal recovery or disposal in landfill. In the event of contamination, applicable laws and national regulations must be observed.
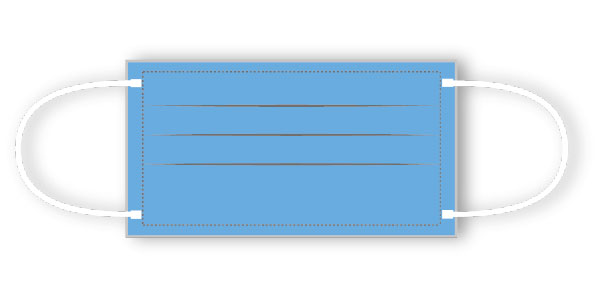
Features
- with nose-bridge
- glass fibre free
- with earloops
- elastic loop round covered with cotton
Product description: Surgical Face Mask
The Face Mask meets the requirements of
DIN EN 14683:2019 for medical face masks / Classification Class Type IIR

|
Requirements of the standard EN 14683:2019 |
TYPE IIR |
|---|---|
|
Bacterial filtration efficiency (BFE) meets the following requirements |
≥ 99,3 % |
| Breathability (Differential pressure) Pa/cm2 |
≥ 19.9 |
| Splash resistance |
Pass |
| Microbial cleanliness (Bioburden) |
≤ 30 CFU/g |
| Material | |
|---|---|
| Inner & external layers | Polyester Fabric |
| Filter material |
Non-woven polypropilene |
| Nose bridge |
Iron wire covered with |
| Ear Loop |
Nylon+Rubber band |
